In 1983, three years before the passage of the National Childhood Vaccine Injury Act (NCVIA), the Childhood Vaccine Schedule included 11 doses of 4 vaccines. The NCVIA effectively removed all product liability from the pharmaceutical companies, and vaccine development kicked into high gear.
As of this writing, the number of vaccines on the CDC-recommended childhood schedule has ballooned to 88 doses of 16 vaccines.
The most recent addition to the Schedule as of this year is COVID-19 vaccine, beginning at 6 months. For the previously unvaccinated, as many as 3 shots of the Pfizer bivalent vaccine or 2 shots of the Moderna bivalent vaccine are authorized, all before the baby has even started crawling.
The COVID-19 dosing schedule will change again this fall when FDA authorizes the latest version of the vaccine that is targeting yet another variant.
As FDA noted in a recent interview,
If authorized or approved, based upon the available evidence, the FDA believes these vaccines with a monovalent XBB.1.5 composition will provide the best available protection against the most serious consequences of the disease resulting from currently circulating variants.
FDA believes? That is hardly reassuring. In a recent substack (Pediatricians Misled on Vaccines) we cover in great detail the flaws and misrepresentations about the soon-to-be-released COVID-19 vaccine.
Public Funding Pushes Vaccination
We expect CDC will push hard to get parents and caregivers to comply with the updated schedule. For example, in 2021, over $3 billion in funding was allocated to states, localities, and jurisdictions through the existing CDC Immunization and Vaccines for Children cooperative agreement.
Over $3 billion will be made available in an initial award to jurisdictions through the existing CDC Immunization and Vaccines for Children cooperative agreement. These awards will support a range of COVID-19 vaccination activities across jurisdictions.
In 2022, CDC spent $680 million to promote childhood vaccines.
That’s a lot of marketing dollars the vaccine manufacturers don’t have to spend on promotion that can fall to the bottom line. Free marketing and no liability risk -what’s not to like?
COVID-19 Vaccines Prompt Another Look at the Vaccine Schedule
Perhaps more than any other vaccine, FDA’s and CDC’s incessant promotion of the COVID-19 vaccine for children has led many to reevaluate the rest of the Childhood Vaccine Schedule.
Despite the reassurances of government agencies and the American Academy of Pediatrics, a closer look at the evidence for the safety and effectiveness of the vaccine schedule raises more questions than answers. We have found the science behind many of the vaccines to be incomplete and in some cases downright misleading.
Gold Standard of Clinical Trials Not Met By Any Pediatric Vaccine
It will come as a surprise to most parents and physicians, as it did to us, that not a single vaccine listed on the pediatric schedule has been subject to a randomized, controlled clinical trial, where the vaccine was compared with a true placebo, such as saline. This is considered the gold standard of clinical trials, yet no pediatric vaccine has met this standard.
Importance of Control Groups During Clinical Trials
Control groups are critical when it comes to assessing safety. Many pediatric vaccines were compared to a previous version of the same vaccine, hardly a neutral control group. Others had follow-up of just days to weeks to detect for any safety signals. (A safety signal is information detected during a clinical trial that may indicate the presence of a serious adverse event potentially caused by a medicine or vaccine and that warrants further investigation).
For example, the pneumococcal vaccine (PCV 15) was tested against a previous version of the same vaccine (PCV 13), which was tested against the original vaccine (PCV7), which was tested against an experimental meningococcal vaccine, but never against an inert placebo like saline.
Safety signals from the latest vaccine may be obscured by comparison to safety signals from earlier versions. Whereas comparison with a placebo with few or no side effects may reveal the true incidence of complications.
The Hepatitis B vaccine (HepB) is an example of inadequate follow-up. HepB, administered the first day of a baby’s life, had only 4 days of safety testing. If a baby in the test group had a serious adverse event after 4 days, it would not be officially attributed to the vaccine.
Pediatric vaccines Engerix-B (HepB), HAVRIX (HepA), and Fluarix (influenza) were never tested for carcinogenesis, mutagenesis, or impairment of fertility. Yet, they are given to our most vulnerable children whose immature immune systems are undergoing rapid development.
Risks Versus Benefits
As with all medications, vaccines should be evaluated based on their risks and benefits for each child. A decision to vaccinate a child should be based on informed consent, the exception in most practices rather than the rule.
Possible Risks include near and long-term adverse events such as allergic reactions, myocarditis, arrhythmias, neuropsychiatric (ADHD, Anxiety) and neurodevelopment disorders (Autism), impaired fertility, cancer, and death (SIDS).
Potential Benefits include decreased incidence of infection and transmission of communicable diseases of significance (high risk diseases), reduced susceptibility to toxins produced by pathogens (e.g. tetanus, diphtheria), decreased likelihood of cancer due to infectious agents (HPV) and building of herd immunity.
Herd Immunity
The latter category of benefits, herd immunity, is a highly desirable characteristic of vaccines in contrast to most pharmaceuticals. In addition to protecting the individual, vaccines hold the potential to protect the vulnerable.
The theory of herd immunity holds that individuals who either can’t take a vaccine due to a health condition or allergy or who don’t respond to a vaccine, will be protected from transmission from those around them who have immunity.
Herd immunity may be achieved when as few as 50% of the population is immunized or has natural immunity, depending on the contagiousness of the disease (the more contagious, the higher the percentage is necessary for herd immunity).
The Optimal Vaccine
The optimal pediatric vaccine therefore is low risk, offers an excellent safety profile (as demonstrated in long-term, controlled clinical trials with a true placebo group for comparison), is highly effective at preventing infection and transmission of diseases of significant risk to healthy children (proven through well-conducted clinical trials), and is capable of inducing herd immunity.
Unfortunately, few pediatric vaccines meet these criteria. We will begin our discussion with the vaccines we feel represent the least benefit in comparison with the potential risks for a general pediatric population under 6 years in good health.
The Risky Six
We have identified six vaccines for children under 6 that merit special attention. They stand out as meeting one or more of the following criteria:
· Benefits do not justify exposure to known or unknown risks
· Diseases prevented are mild, exceedingly rare or have effective treatments
· Immunity wanes over time, exposing the vaccinated to more serious infection as they age
· Impaired immune function
· No effect on herd immunity
· Risk of autoimmune disorders, neurocognitive disability (autism), or SIDS (to be covered in a future Substack article)
The Risky Six include:
COVID-19
Hepatitis B
DTaP
MMR
Varicella (chicken pox)
Influenza
In Part One, we review two of the six vaccines with the worst risk to benefit ratios for the vast majority of children: COVID-19 and Hepatitis B.
COVID-19 Vaccine
We have written extensively about the risks versus limited benefits of COVID-19 vaccine for healthy children (here, here, here). The mRNA-based boosters proposed for this fall offer little protection, will not prevent infection or spread, and pose a serious risk of harm. Asymptomatic children are simply not vectors for the disease.
For example, a study published in Nature found no instances of asymptomatic spread from PCR-positive but asymptomatic cases based on a sample of 10 million persons. A Swedish study of nearly 2 million children had similar findings.
Adverse Events
Of much greater concern are the adverse events due to the vaccine such as myocarditis, pericarditis, neurological disorders, autoimmune disorders, and immune system impairment.
Recently, a study of children before and after their second Pfizer COVID-19 vaccine found a marked decrease in immunity to different types of infections such as bacteria and fungi as well as other viruses. This decreased immunity to other pathogens (acquired immune deficit) is referred to by some as “VAIDS” (Vaccine Acquired Immunodeficiency Syndrome). Immune system effects were present 6 months out when they stopped measuring and may last for years.
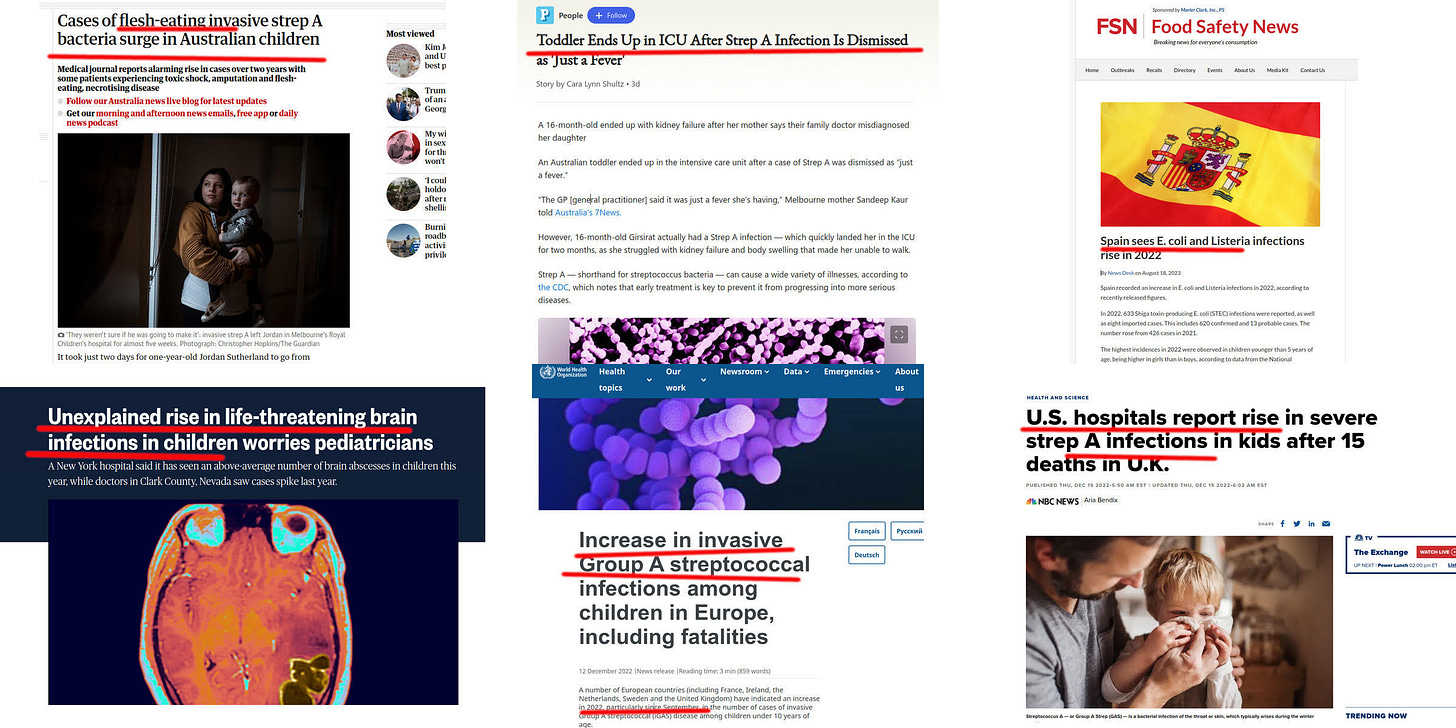
Reports of outbreaks (and here) of potentially deadly Staphylococcus and streptococcus infections may have VAIDS as a contributing factor, as the rise in infections correlates with vaccinations, not COVID-19 infections.
The CDC said invasive strep A infections went down during the COVID-19 pandemic. They began to rise in 2022 and 2023, and they’ve stayed high in some parts of the country.
Widespread Natural Immunity in Children
Over 90% of US children have antibodies to COVID, and over two-thirds of those have acquired natural immunity, which is robust and enduring. In stark contrast, a study of nearly 900,000 children aged 5-11 published in the New England Journal of Medicine shows that mRNA vaccine effectiveness rapidly deteriorates over a few weeks and becomes negative over time (meaning vaccination actually increases risk of infection).
The CDC just cautioned that the vaccinated are more likely to get infected with the latest variant this fall. Negative efficacy day one? Good to know.
Worse still, the vaccine reduces protection gained from natural immunity, as we described in our July 19, 2022 substack post.
COVID-19 Vaccine Safety Largely Ignored by CDC and FDA
Nor does the government seem terribly interested in learning about vaccine adverse events that will inevitably occur as new vaccines are released.
As we noted in our August 15 newsletter, Pediatricians Misled on Vaccines, CDC’s own monitoring system “V-safe,” was developed specifically for monitoring the safety of the COVID-19 vaccines.
The program, which had enrolled 10 million people to self-track vaccine reactions, shut down this summer after discovering a dangerously high 7.7% incidence of potentially life-threatening, severe adverse events.
The government didn’t like the results, so they simply stopped keeping track.
DNA Contamination and Variable Batch Quality
Reports of DNA contamination (DNA plasmids left over from the production process) and production batch variability of mRNA vaccines continue to be ignored by FDA. Perhaps the most alarming finding was the presence of SV40 cancer promoter gene in the DNA contaminant. Not surprisingly, some batches of vaccines have been associated with a disproportionate number of adverse events.
Emergency Use Authorization Versus Full Approval
New versions of COVID-19 vaccines have been released under Emergency Use Authorization (EUA) and not full approval based on a Biologics License Application (BLA). FDA has at times tried to blur the differences between the two.
However, there are important distinctions between what is required of manufacturers that submit applications for an EUA versus a BLA that bear on the potential safety, production quality, and effectiveness of any vaccine.
Two stand out.
First, for BLA approval the FDA wants to see longer follow-up of trial participants, particularly at least six months of safety data versus minimal to none (Pfizer using mice models) for the boosters to be released this fall.
Second, the FDA needs more detailed chemistry, manufacturing, and control data (including requiring facility inspections) for BLA approval. The agency has indicated that working through this vast amount of data takes months.
Keep this in mind as FDA reels off one EUA after another for new COVID-19 vaccines.
COVID-19 Infection Low Risk to Children
Recall one of our essential criteria is that vaccines should be reserved for prevention of diseases that pose a serious threat The most recent data by the American Academy of Pediatrics showed that “Children were 0.00%-0.19% of all COVID-19 deaths, and 10 [US] states reported zero child deaths. In states reporting, 0.00%-0.03% of all child Covid-19 cases resulted in death.” There are no reported deaths attributed directly to COVID-19 in a healthy child. None.
COVID-19 morbidity and mortality pose only a minor risk to healthy kids and fail to justify the considerable risk these novel mRNA vaccines represent to children.
Summary COVID-19 Vaccine
Clinical Trials
Approved under EUA with limited clinical testing. No randomized, controlled, blinded clinical trials for mRNA boosters to be released this fall. Pfizer EUA based on mouse studies; Moderna EUA based on human antibody response, not clinical efficacy trials. No long-term safety studies or testing for carcinogenesis or mutagenesis.
Risks
Novel technology, inadequately tested. High incidence of side effects, some of which are life-threatening. Toxic lipid nanoparticles (LNPs) containing modRNA (not mRNA) distribute widely in the body, are not easily broken down, and impair immune system response to a variety of diseases. May increase risk of contracting new variants.
Benefits
Does not prevent infection or transmission. COVID-19 is a benign illness in most healthy children. There is already widespread natural immunity among children. Does not lead to herd immunity.
Assessment - GRADE F
Risks far outweigh Benefits in healthy children. High potential for serious adverse events, including long-term immune system impairment.
Hepatitis B (Engerix-B)
Hepatitis B is the most common serious liver infection in the world. It is caused by the hepatitis B virus (HBV) and can result in potentially fatal cirrhosis and liver cancer. Most people who get infected clear the virus naturally, although a small percentage become chronic carriers and can transmit the disease.
Fortunately, HBV is not easy to catch as with measles or chickenpox where simple proximity can result in infection. HBV is typically only transmitted through direct contact with infected blood or certain bodily fluids, such as through sharing unsterile needles, contaminated medical equipment or unprotected sex.
Who Is At Risk of HBV?
The key risk factors for HBV infection are well-known. These categories do not indicate that a person is infected, nor does it assure those without risk factors are not infected. Most infections are sexually transmitted. The only way to tell for certain is a blood test.
Generally accepted risk factors include injection drug use, more than 5 sexual partners per year, sex workers, men having sex with men, HBV positive household contacts, developmentally disabled living in residential facilities, prison inmates, hemodialysis patients, occupational exposure (medical, dental), frequent travel to HBV-endemic countries, hepatitis C infection, chronic liver disease, HIV infection, diabetics.
HVB Transmission During Birth
An infected mother can transmit the virus to their baby during childbirth, although the baby is protected during gestation. A simple blood test can detect HBV infection, and the vast majority of pregnant women are screened during pregnancy.
In the US, chronic carriers who might infect their infants are quite rare, estimated at 0.3% (3 per 1000 or 1 in 333), although the prevalence of carriers among inner-city African American and Hispanic women (0.4%-1.5%) is much higher.
Children born to a chronic carrier are at very high risk of contracting the disease and becoming chronic carriers themselves. Therefore, prevention through adequate pre-natal testing and Hepatitis B (HepB) immunization is essential in high-risk pregnancies. HepB vaccine does contribute to herd immunity and is very effective at preventing infection.
But does every child need to be vaccinated immediately after birth if low-risk mothers test negative? And how safe is the HepB vaccine?
Clinical Trials
According to the FDA approved package insert,
In 36 clinical studies, a total of 13,495 doses of ENGERIX-B were administered to 5,071 healthy adults and children who were initially seronegative for hepatitis B markers, and healthy neonates. All subjects were monitored for 4 days post-administration.
Wait, what? There was a total of 36 clinical studies conducted on thousands of healthy adults and children and all had follow-up limited to 4 days.
How about a control group? Well, Engerix-B was compared to a previous generation product, a plasma-derived vaccine called Heptavax, and not to a true placebo. As could be expected, adverse reactions limited to the first few days were mostly soreness and fatigue.
Voluntary Reporting of Adverse Events High
Post-marketing (meaning post-approval) adverse events using the voluntary VAERS reporting system tell a different story. During 2005–2015, a total of 20,231 reports of adverse events following HepB vaccination among all ages were submitted to VAERS. The majority of primary U.S. reports (15,787 of 20,231, 78%) were following HepB vaccine administered with other vaccines on the same visit.
However, among the 4,444 single-dose HepB-related reports, 6.5% were classified as serious, including 43 deaths, of which 27 were among infants aged less than 4 weeks.
What other post-marketing adverse events have been reported?
According to the manufacturer’s (pharmaceutical giant GlaxoSmithKline) package insert which must disclose the known complications, reported adverse events include among other things, meningitis, encephalitis, herpes zoster, anaphylaxis, hypersensitivity syndrome (a potentially fatal autoimmune disorder), arthritis, multiple sclerosis, neuropathy, Guillain-Barré syndrome and Bell’s palsy, seizures; syncope; and transverse myelitis (inflammation of the spinal cord that can lead to paralysis).
The package insert notes,
…because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
True enough, but a Harvard vaccine injury study found that fewer than 1% of vaccine adverse events are reported to the VAERS system. The fact that so many catastrophic neurological complications were picked up by a voluntary tracking system raises enormous questions about HepB vaccine safety.
Effective but Unnecessary for Most
In summary, HepB vaccine is extremely effective at prevent peri-natal transmission of HepB to the newborn. However, there has been no randomized, placebo-controlled clinical trial to assess the safety of HepB vaccination to newborns and infants. None.
It can be argued that to withhold an effective vaccine from a high-risk infant is unethical. Fair enough. But a control group injected with saline placebo could be easily constructed from children of HBV-negative mothers who are at no-low risk of the disease.
Routine HepB vaccination of all children is as much a political decision as it is a public health decision. It is the public health community’s effort to appear non-discriminatory against those persons and communities at highest risk.
So all children get the shots, even though only 1 in 333 children are at risk, and the higher risk newborns can be screened for.
Mothers at low risk who test negative should be given the option not to vaccinate or delay vaccination of their children unless or until they or their children subsequently fall into a high-risk category or regular caregivers test positive for HepB.
The delay of vaccination in a newborn by a month could have a profound effect on neurodevelopment. Results of a study never released by the CDC, a copy only obtained under a FOIA request, found that children vaccinated in the first month of life compared with children without vaccination had 2 times increased risk for speech delays, five times increased risk for sleep disorders, and 7.6 times increased risk for autism.
If in doubt, vaccinate. But if a mother is low risk and tests negative for HBV, vaccination risks clearly outweigh the benefits.
Summary Hepatitis B Vaccine
Clinical Trials
No randomized, placebo-controlled clinical trial. Short term follow-up only. Comparison group limited to a previous vaccine. No long-term safety studies in children for carcinogenesis, mutagenesis, or fertility.
Risks
Evidence for serious and even fatal neurological complications. Safety assessed only in comparison with a previous vaccine, not placebo.
Benefits
Only for those at risk. Greater than 99.6% of children do not benefit yet are exposed to vaccine risks. Does provide some measure of herd immunity. But herd immunity is best achieved by avoiding risk factors, not vaccinating those at no or low risk.
Assessment - GRADE F
Risks without benefits in healthy children. For most, this vaccine is largely for equity, not health.
Coming Next
In Part Two we will discuss the remaining four of the Risky Six including Tdap, MMR, Varicella, and Influenza vaccines. Get ready for a deep dive into vaccines, autism and SIDS.
FLASHBITS