In our recent book, Unavoidably Unsafe: Childhood Vaccines Reconsidered, Dr. Jeff Barke and I went to great lengths to explain the risks and benefits of vaccines on the CDC’s Childhood Vaccination Schedule. We rated each vaccine to allow parents and caregivers alike the chance to evaluate the appropriateness of each vaccine for any particular child. We linked our assessments to primary sources for those wishing to probe more deeply into the evidence we considered.
Our goal was to enable true Informed Consent, freely given or withheld based on what families determine is in their child’s best interests.
Since publication, we have spoken with hundreds of parents, grandparents, and providers in various settings about our recommendations. And we have continued our search for information that might shed additional light on this controversial subject. We are troubled by what we found.
Vaccine Moratorium and New Standard of Informed Consent
As a result, we have arrived at a difficult, but we think inevitable conclusion: it is time for a childhood vaccine moratorium until they can be properly studied for safety and effectiveness in randomized, placebo-controlled, clinical trials or their equivalent.
Pending such testing, childhood vaccines should be administered on an exception, not routine basis. We call this Vaccination by Exception, necessitating a new standard of Informed Consent. As we describe below, this means that the burden of proof is shifted to the caregiver to make the case to parents and guardians that a particular vaccine is both safe and effective for a child.
What Changed Our Minds?
Some of the factors that led to our decision are well-covered in our book. These include:
Despite a dramatic increase in the number of vaccines the health of American children is in sharp decline, including chronic illness and immune disorders such as asthma, eczema, allergic rhinitis, diabetes, arthritis, and lupus.
Serious illness from many communicable diseases was in decline before the introduction of vaccines
Regulatory capture, where agencies (FDA, CDC) responsible for protecting public health are compromised by the very industries they are supposed to regulate
Revolving door between government officials and private industry – for example, 9 of the last 10 FDA Commissioners left to join pharmaceutical companies
Conflicts of interest among the “independent” advisors to the FDA and CDC regarding new vaccine approvals
Federal legislation that protects vaccine manufacturers from product liability
A lax regulatory structure that compromises vaccine approvals and safety testing
Absence of randomized, controlled clinical trials in support of vaccine safety and efficacy
Failure to properly analyze and report on vaccine adverse events data obtained through VAERS and VSDL (Vaccine Safety Data Link)
Failure to act on information collected during post-marketing surveillance required of vaccine manufacturers
Arbitrary declaration of a public health emergency that authorizes vaccine approvals under an Emergency Use Authorization (EUA)
EUAs do not require any clinical trials or vaccine safety testing prior to release into the public
Three Additional Factors That Drove Our Call for a Moratorium
The rapid pace of novel vaccine technology development (e.g. mRNA pipeline, monoclonal antibodies) exceeds the ability or willingness of regulators to provide proper oversight
Lack of safety testing for aluminum and other vaccine adjuvants and failure to adequately study an increasing number of vaccine combinations
Growing evidence for a link between aluminum adjuvants and autism spectrum disorder (ASD).
Failure of regulatory agencies to disclose data that contradicts their public health advocacy positions (e.g., vaccinated vs. unvaccinated comparison data, post-marketing surveillance data).
Rapidly Evolving Vaccine Technology
The pace of change in the field of vaccinology warrants a pause. Novel vaccine technologies are emerging that are not well understood. The use of EUAs poses the additional risk of inadequate safety testing before mass distribution.
For example, the mRNA vaccines were never tested for biodistribution (meaning where they go in the body after injection – a standard test for all drugs) only to find out afterward they go everywhere including the brain, heart, and reproductive organs tissue.
Manufacturers see vaccines as a virtual fountain of commercial opportunity, with the potential to far surpass drug revenue. Each vaccine added to the Childhood Vaccination Schedule assures the manufacturer of $1B to $2B or more on average in annual recurring revenue – all without any liability for the company.
Leveraging these novel and largely untested technologies to more rapidly prototype and develop vaccines opens the field to tremendous innovation as well as risk.
We discuss the extraordinary risks posed to children from some of these novel technologies in Chapter 17 of our book titled, “Why A Child Should Never, Ever Be Given an mRNA Vaccine.”
PREP Act
Permitted under the 2005 PREP Act, Emergency Use Authorization (EUA) enables rapid development and approval of novel vaccine technologies without proper clinical safety and effectiveness testing.
For example, there is no requirement for any human clinical safety testing before releasing a vaccine under an EUA. If the Director of Health and Human Services declares a public health emergency, he or she can authorize releasing vaccines or other medical “countermeasures.”
One need not look further than the mRNA vaccine platform first authorized for use under an EUA in December 2020 to find a potential Pandora’s box of dangers. As we discussed extensively in our book, mRNA technology leverages genetic engineering techniques to synthesize vast quantities of novel vaccines in the lab, replacing the laborious and expensive process of culturing vaccines in eggs or cell cultures.
This has led to FDA’s recent EUA for a 3-shot Pfizer (2-shot Moderna) COVID-19 mRNA vaccine series intended for children as young as six months of age. This authorization came despite no reported clinical safety or effectiveness data for children under 12.
Several other childhood mRNA vaccines are in the pipeline, including for RSV, influenza, new COVID-19 strains, and combination vaccines, which will likely be released under an EUA.
Self-Replicating Vaccines
Expanding on existing mRNA technology, manufacturers are ready to deploy self-amplifying mRNA vaccines or sa-mRNA that can replicate themselves inside each person’s cells. Japan has already approved for one such product for COVID-19, CSL and Arcturus Therapeutics' sa-mRNA vaccine named ARCT-154.
We view sa-mRNA technology as particularly troubling, as it enables the mRNA to replicate itself once it enters a cell. This allows the mRNA to continuously produce foreign proteins inside of each cell.
When and if the sa-mRNA ever shuts itself off is completely unknown, posing enormous potential risks for auto-immune disorders, immune imprinting (lowered immune response to new variants), antibody-dependent enhancement (where the vaccine actually facilitates the infection it is intended to prevent), and other toxic effects.
And the innovation continues. Frustrated that mRNA technology cannot keep up with SARS-CoV-2 mutations, Big Pharma continues its search for new COVID-19 technologies (even though the virus continues to weaken as it mutates). One such effort involves using self-assembling nanoparticles that can contain multiple viral antigens simultaneously. Such growing complexity of vaccine technology mandates more, not less safety testing of vaccines before FDA authorization.
FDA Claims Aluminum Adjuvants Are Safe – Where is the Evidence?
Aluminum compounds are added to vaccines to stimulate an immune response to the target antigens. Such compounds are called adjuvants. Absent aluminum adjuvant stimulation, immature immune systems in early childhood may fail to develop immunity from the vaccine. This is in contrast to actual infection during childhood which typically results in enduring, natural immunity.
The government has repeatedly claimed that aluminum adjuvants are safe. FDA states,
Aluminum adjuvant containing vaccines have a demonstrated safety profile of over many decades of use and have only uncommonly been associated with severe local reactions.
Despite safety claims, the government has failed to provide evidence supporting that conclusion. In 2019, the Informed Consent Action Network (ICAN) submitted a Freedom of Information Act (FOIA) request to the CDC asking for their supporting data. After much back and forth over the next 4 years, the CDC conceded it had no such evidence for the safety of aluminum adjuvants in vaccines.
ICAN’s attorneys sent the same request to the NIH, which also conceded that no records were found.
As summarized by ICAN:
CDC and NIH’s responses to ICAN’s Freedom of Information Act (FOIA) requests regarding aluminum adjuvant reveal a stunning admission: they do not have a single study to support the safety of recommending repeated injection of this cyto- and-neuro toxic substance as part of the CDC’s childhood vaccine schedule.
It’s worth noting that aluminum is one of more than 35+ adjuvants and vaccine additives. Only one has been studied by FDA, thimerosal, which was removed from most vaccines in 2001 (still present in some multidose flu vaccines).
Growing Evidence for an Association Between Aluminum Adjuvants and Autism Spectrum Disorder
Beyond the rapid pace of vaccine innovation, we continue to uncover more evidence for nervous system toxicity caused by vaccine aluminum adjuvants and an emerging link between adjuvants and Autism Spectrum Disorder (ASD).
Evidence for aluminum adjuvant neurotoxicity and association with ASD includes the following observations:
Injected aluminum adjuvants are poorly soluble particles transported throughout the body, including the brain. This stands in contrast to ingested aluminum which is rapidly eliminated in feces or by the kidneys. The build-up of aluminum in the brain appears to be associated with repeated exposure to aluminum adjuvants (see Here, here, and here).
High levels of aluminum are found in autistic brains on post-mortem examinations.
Aluminum in the brain is highly inflammatory, triggering the production of inflammatory cytokines (interleukin 6 or IL-6) and neuroglial activation (neuroglia are cells that support and protect neurons) (Here, here, and here).
The association of brain inflammation with autism spectrum disorder. An excellent summary of the available evidence is here.
A New Standard for Informed Consent for Childhood Vaccination
We believe that a new standard for Informed Consent is required, one that shifts the burden of proof to the providers to convince parents that vaccines are both safe and effective. Vaccines should not be considered routine, rather, the need for each vaccine should be carefully evaluated and documented before a decision is made to vaccinate a child.
We propose that a minimum set of criteria be met to justify vaccination. Informed Consent should include documentation of the following factors for each child:
Elevated risk of infection (e.g. underlying sickle cell disease, cystic fibrosis, impaired immune system, cancer, HepB+ mother, TB exposure, etc.)
If infected, at high risk of serious illness (can be expected to develop more serious illness compared to other children due to an underlying condition)
Absence of preexisting immunity or prior history of infection (no natural immune system protection)
No effective therapies or other preventive measures, including off-label use of medications (all alternative therapies should be considered)
No known or suspected contraindications to the vaccine or excipients (allergies, etc.)
Review of vaccine risks and benefits as provided in the FDA-approved vaccine package insert (CDC’s Vaccine Information Statement (VIS) considered inadequate as a baseline source of information)
Only after review and discussion of this information with the provider should a parent or guardian authorize (or withhold) written Informed Consent on behalf of the child.
Conclusion
We believe the balance of evidence now supports an immediate halt to childhood vaccination pending proper safety and efficacy testing. That said, we recognize that it is difficult to reverse decades of vaccination culture for the public, public health agencies, and providers regardless of how compelling or powerful the evidence may be for a moratorium.
As a result, we propose instituting a Vaccine by Exception strategy in contrast with a regular vaccination schedule, so that the burden of proof shifts to manufacturers, regulatory agencies, and providers to demonstrate the safety and effectiveness of vaccines for each child.
We call for a new standard of Informed Consent that meets a minimum set of criteria in order to obtain written approval from parents or guardians on behalf of the minor before administering a vaccine.
The current vaccine consent process is far too cursory and lacks sufficient information to enable true Informed Consent. Safety is assumed and only potential benefits are considered. Far too little attention has been paid to devastating vaccine injuries and chronic illnesses that may be the result of repeated vaccination during a child’s formative years.
Personal vaccine exemptions should prevail over state mandates during the moratorium and all mandatory vaccinations should be eliminated.
It's time to slow down the vaccine train that keeps adding more and more vaccines to the childhood schedule until the public can regain confidence in the safety and effectiveness of any particular vaccine for their child.





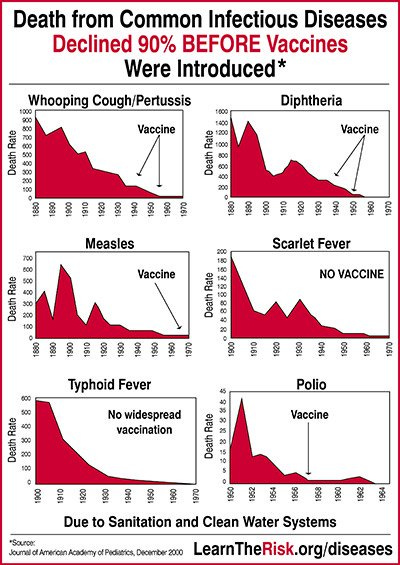
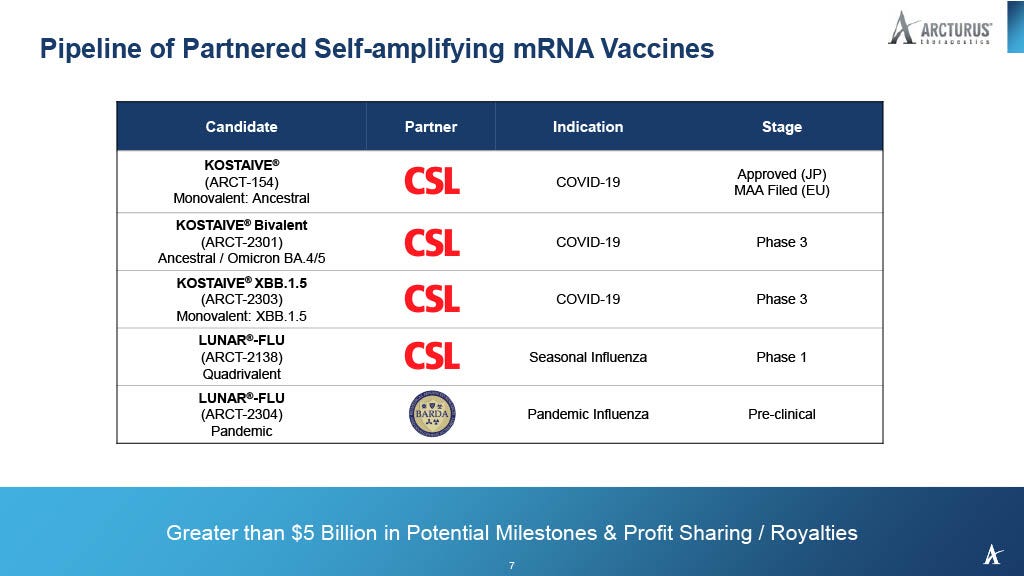
Even under the best of circumstances, vaccines will never approximate the protection afforded by natural immunity and will always induce a toxic immune response.
This article needs to be spread far and wide, especially to parents of young children and would-be parents. Too often, they are way too trusting when it comes to this. They all grew up injected multiple times and think all this has been thoroughly investigated, which it is NOT. Thank you for posting this.